Why is the Grass Green?
I was outside talking with Alemi last week and we were both startled to
realize that the frozen white tundras of Ithaca had somehow transformed
into fields of green. Apparently the snow was a temporary fixture that
covered real live grass. Neato, gang! The joy at seeing green grass led
quickly to surprise, confusion and then anger. Why the heck is grass
green? Well, things look a color when they reflect back that color. So
grass is green because its pigments (chlorophyll) absorb only a certain
range of the visible spectrum, reflecting back the greenish bits. But if
I know anything about approximating the sun as a blackbody, I know that
it has a peak output of around 550 nm (i.e. green) light. So what's
going on? Why are plants blatantly rejecting the most abundant kind of
light? Since my initial confusion rested on the assumption of the sun as
a blackbody, I decided to take a closer look at the actual spectrum of
the sun. Below is a graph showing the frequency dependence of solar
radiation incident on the top of the atmosphere and at sea level. Since
plants typically don't live in space, we are most concerned with the sea
level plot.
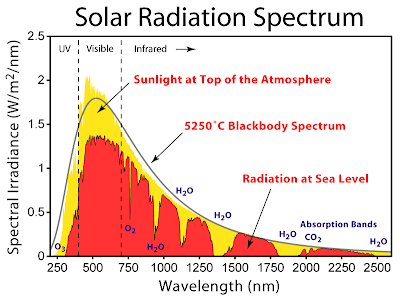 From this plot, it looks like the incident radiation from the sun is
fairly level beyond about 450nm or so. Just going on this graph alone,
it looks like plants could just absorb reddish light and do alright for
themselves. But do they? Let's take a look at the absorption spectrum of
chlorophyll. As it turns out there are a bunch of different "flavors" of
chlorophyll (chlorophyll a, b, c, and d). As far as I could gather, only
a and b are important (a is found in just about everything that plays
the photosynthesis game and b is found in plants and green algae). So we
need to find the absorption spectrum of chlorophyll a and b. After
looking for a while at very qualitative drawings, I found this
neato-toledo
site, which
actually gives real live data. Plotting the results, we get the figure
below.
From this plot, it looks like the incident radiation from the sun is
fairly level beyond about 450nm or so. Just going on this graph alone,
it looks like plants could just absorb reddish light and do alright for
themselves. But do they? Let's take a look at the absorption spectrum of
chlorophyll. As it turns out there are a bunch of different "flavors" of
chlorophyll (chlorophyll a, b, c, and d). As far as I could gather, only
a and b are important (a is found in just about everything that plays
the photosynthesis game and b is found in plants and green algae). So we
need to find the absorption spectrum of chlorophyll a and b. After
looking for a while at very qualitative drawings, I found this
neato-toledo
site, which
actually gives real live data. Plotting the results, we get the figure
below.
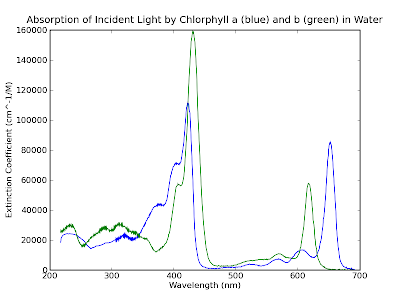 Comparing with our handy dandy wavelength-to-color converter below we
see that there is a big peak in absorption of both chlorophyll a and b
in the dark blue and lesser peaks in the red.
Comparing with our handy dandy wavelength-to-color converter below we
see that there is a big peak in absorption of both chlorophyll a and b
in the dark blue and lesser peaks in the red.
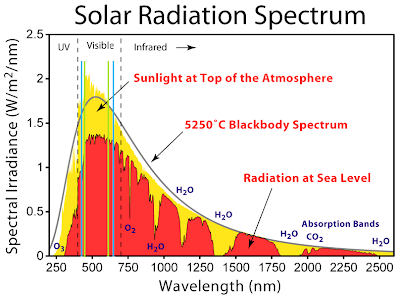
 So how do these absorption lines correspond to the incident light at sea
level given above. Well, its kind of tough to check by eye, but it looks
like chlorophyll has its biggest peaks right below the plateau in the
solar spectrum. Marking the wavelengths of the absorption peaks makes
this clear (a = blue, b = green). It seems like plants are using a
sub-optimal band of the spectrum!
So how do these absorption lines correspond to the incident light at sea
level given above. Well, its kind of tough to check by eye, but it looks
like chlorophyll has its biggest peaks right below the plateau in the
solar spectrum. Marking the wavelengths of the absorption peaks makes
this clear (a = blue, b = green). It seems like plants are using a
sub-optimal band of the spectrum!
 So how do we reconcile this? Well, let's first start at The Beginning.
The first photosynthetic organisms developed and spent the first billion
or so years of their existence living and evolving in the oceans. The
solar spectrum we have been using so far has been accurate only in air
at sea level. Presumably it would be favorable for the organism to be
able to survive at some finite depth in the ocean and not merely at the
surface. Thus we must consider the effects of water on our incident
solar radiation. A plot of the absorption spectrum of water is shown
below (note the log-log scale).
So how do we reconcile this? Well, let's first start at The Beginning.
The first photosynthetic organisms developed and spent the first billion
or so years of their existence living and evolving in the oceans. The
solar spectrum we have been using so far has been accurate only in air
at sea level. Presumably it would be favorable for the organism to be
able to survive at some finite depth in the ocean and not merely at the
surface. Thus we must consider the effects of water on our incident
solar radiation. A plot of the absorption spectrum of water is shown
below (note the log-log scale).
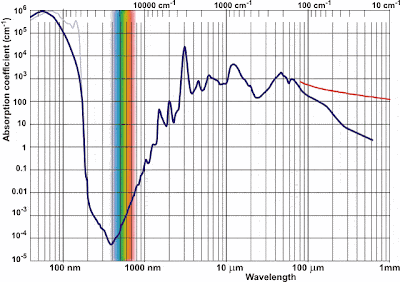 Lo and behold, the minimum absorption of visible light in water occurs
towards the far end in the blue. And this is exactly where our biggest
absorption peak in of chlorophyll is! Comparing our two graphs we see
that the ratio of incident blue light to incident green light at see
level is at worst about a third. But we see that for each meter traveled
in water, green light is absorbed almost ten times more than blue light.
Thus, an organism that lives a few meters underwater and wants to
harness solar energy would probably do best to focus on that blue light.
[WARNING: The next bit is speculative and I haven't taken a bio class
since high school] As much as I gather about chlorophyll from Wikipedia
and other semi-reputable sites, chlorophyll a is found in just about
anything that photosynthesizes, BUT chlorophyll b is only found in
plants and green algae. And apparently, land plants are largely
descended from green algae (which are aquatic, but typically around the
surface and around the shoreline). Now take a look at the chlorophyll
absorption spectra again. The chlorophyll b spectrum is sort of squished
in more towards the middle (towards 550 nm maybe?) than chlorophyll a.
In fact, on the graph where I have drawn lines on the solar spectra
graph where the chlorophyll peaks are, we see that chlorophyll b just
barely gets up to that plateau region. This suggests to me that
chlorophyll a was working just fine for the early aquatic plants, but
once they reached land and got out of all that water it became an
advantage to utilize light closer to the peak solar output. Thus, plants
that had chlorophyll b in addition to a had a slight advantage over
their b-less brethren. Or so I shall continue to shamelessly speculate
(and, apparently, alliterate). Anyway, I thought that was kind of cool,
but if I have made some horrible error or mangled some biology, please
let me know!
Lo and behold, the minimum absorption of visible light in water occurs
towards the far end in the blue. And this is exactly where our biggest
absorption peak in of chlorophyll is! Comparing our two graphs we see
that the ratio of incident blue light to incident green light at see
level is at worst about a third. But we see that for each meter traveled
in water, green light is absorbed almost ten times more than blue light.
Thus, an organism that lives a few meters underwater and wants to
harness solar energy would probably do best to focus on that blue light.
[WARNING: The next bit is speculative and I haven't taken a bio class
since high school] As much as I gather about chlorophyll from Wikipedia
and other semi-reputable sites, chlorophyll a is found in just about
anything that photosynthesizes, BUT chlorophyll b is only found in
plants and green algae. And apparently, land plants are largely
descended from green algae (which are aquatic, but typically around the
surface and around the shoreline). Now take a look at the chlorophyll
absorption spectra again. The chlorophyll b spectrum is sort of squished
in more towards the middle (towards 550 nm maybe?) than chlorophyll a.
In fact, on the graph where I have drawn lines on the solar spectra
graph where the chlorophyll peaks are, we see that chlorophyll b just
barely gets up to that plateau region. This suggests to me that
chlorophyll a was working just fine for the early aquatic plants, but
once they reached land and got out of all that water it became an
advantage to utilize light closer to the peak solar output. Thus, plants
that had chlorophyll b in addition to a had a slight advantage over
their b-less brethren. Or so I shall continue to shamelessly speculate
(and, apparently, alliterate). Anyway, I thought that was kind of cool,
but if I have made some horrible error or mangled some biology, please
let me know!
Comments
Comments powered by Disqus